Describe the Roles of Reactants and Products in Chemical Reactions
A reactant is a substance that is present at the start of a chemical reaction. A how enzymes affect reaction rates B how enzymes affect activation energy C how enzymes interact with reactants and how D activators and competitive and non-competitive inhibitors interact with enzymes.

Catalyst Speeds Up A Chemical Reaction By Lowering The Activation Energy Ea Of The Overall Reaction Chemistry Education Teaching Chemistry Energy Activities
Products are the chemical species that can be found after the completion of the reaction.
. Reactants are substances that start a chemical reaction. Reactants and products are the two major components of a chemical reaction. A Chemical Reaction is a process that occurs when two or more molecules interact to form a new products.
Your body lives and grows thanks to chemical reactions. Products are substances that are produced in the reaction. Predict the products of simple reactions given the reactants.
In chemical reactions atoms which are the building blocks of reactants are rearranged to develop new entities referred to as products. Basic Concepts of Chemical Reactions. The substances that start a chemical reaction are called reactants.
Here is an example of a reaction called combustion. Click Create Assignment to assign this modality to your LMS. Thus for a chemical reaction in an isolated device the mass continues to be constant.
They are known as reactants. The first one the products are obtained when the reactants are changed. Chemical changes are chemical reactions.
Reactants are the starting material of a chemical reaction. The substances may change as the elements within them may recombine into new substances. The substances used in the beginning of a chemical reaction are called the reactants usually found on the left side of a chemical equation and the substances found at the end of the reaction are known as the products usually found on the right side of a chemical equation.
The materials involved in a chemical reaction. Matter interacts to form new products through a process called a chemical reaction or chemical change. Describe the role of enzymes in chemical reactions in detail.
During a chemical reaction the substances that react are known as reactants whereas the substances that are formed during a chemical reaction are known as products. The products are carbon dioxide gas and water vapor. Physical changes can be breaking into smaller parts or phase changes.
The substances that form as a result of a chemical reaction are called products. During a chemical reaction the atoms are rearranged. It is the calculation of quantitative measurable relationships of the reactants and products in a.
Answer 1 of 8. A chemical reaction is when two or more substances combine or interact. Equation for endothermic reaction Reactants Heat - Products.
And contrast types of chemical reactions. Classify reactions as synthesis decomposition single replacement double replacement or combustion. The production of new substances from one or more chemical species.
There are reactions when you take medications light a match and draw a breath. How do the reactants and products determine the type of. Limiting reactant is the reactant which defines the amount of product as a a result of.
All chemical reactionsincluding a candle burninginvolve reactants and products. The process of change of one element into another is called chemical reaction. The law of conservation of matter by balancing.
Identify evidences that indicate a chemical reaction has taken place. Products and reactants are substances. Products are the substances that form as a result of chemical reactions.
Different Types of Chemical Reactions Combination Decomposition Combustion Neutralization Displacement Reactions. When a candle burns the reactants are fuel the candlewick and wax and oxygen in the air. Balance chemical equations by applying the Law of Conservation of Matter.
The law of the conservation of masses states that matter deserve to neither be produced nor damaged. Cellular respiration is the process responsible for converting chemical energy and the reactantsproducts involved in cellular respiration are oxygen glucose sugar carbon dioxide and water. It uses oxygen from the air and cellulose from the match as the reactants.
The products of a chemical. There are various types of chemical reactions such as acid-base reactions redox reactions and combustion reactions. A chemical reaction in equation form.
Six common types of chemical reactions are discussed below. Identify the roles of reactants and products in chemical reactions. Solids liquids and gases.
Reactants and products in a chemical reaction is stoichemistry. Defines reactant product and chemical equation. During chemical reactions the reactants are used up to create the products.
Reactants and Products Chemical reactions make and break the chemical bonds that hold atoms together to create molecules. The reactants are transformed during the chemical reaction into products. For example when methane burns in.
Every time you cook or clean its chemistry in action. Use relevant course terminology and explain. Compounds that interact to produce new compounds are called reactants whereas the newly formed compounds are called products.
While the exact steps involved in cellular respiration may vary from species to species all living organisms perform some type of cellular respiration. 3 41 Chemical and physical change Transformation of atoms and molecules from one form to another is classified as either a physical or chemical change. Reactants are the substances that start chemical reactions.
The substances to the right of the arrow are called products. The substances to the left of the arrow in a chemical equation are called reactants. During the reaction the reactants are used up to create the products.
Chemical reactions play an integral role in different industries customs.

What Is The Role Of A Solvent In A Chemical Reaction

Biochemical Reactions Ck 12 Foundation

Ch103 Chapter 7 Chemical Reactions In Biological Systems Chemistry

Chemical Reactions Types Definitions And Examples Teaching Chemistry Chemistry Classroom Chemistry Notes

Pictorial Representation Of The Five Types Of Chemical Reactions Nicole Galante Teaching Chemistry Chemistry Classroom Chemical Reactions
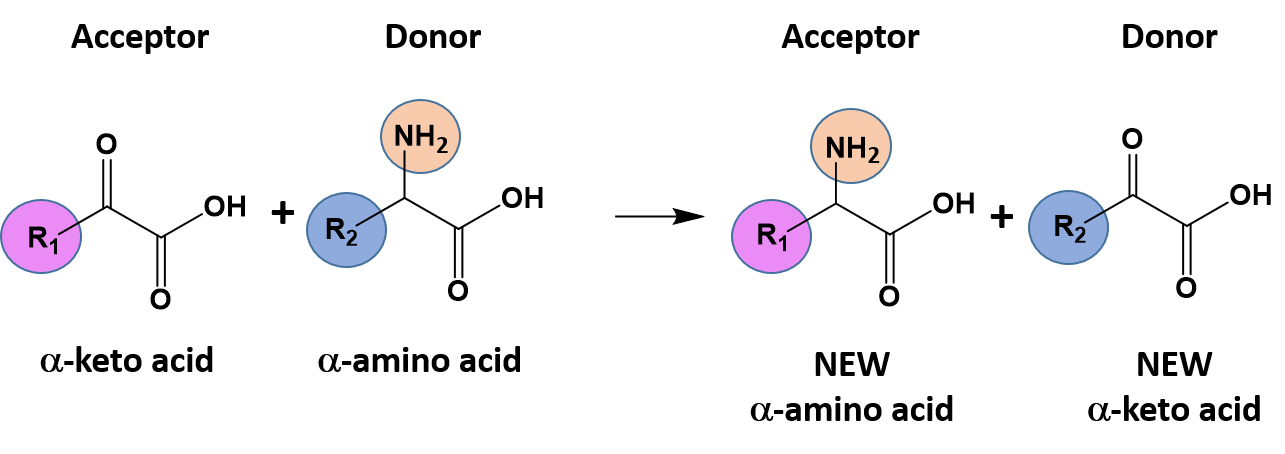
Ch103 Chapter 7 Chemical Reactions In Biological Systems Chemistry

Balancing Chemical Equations Pogil Handwriting Worksheets For Kindergarten Chemical Equation Chemistry Worksheets
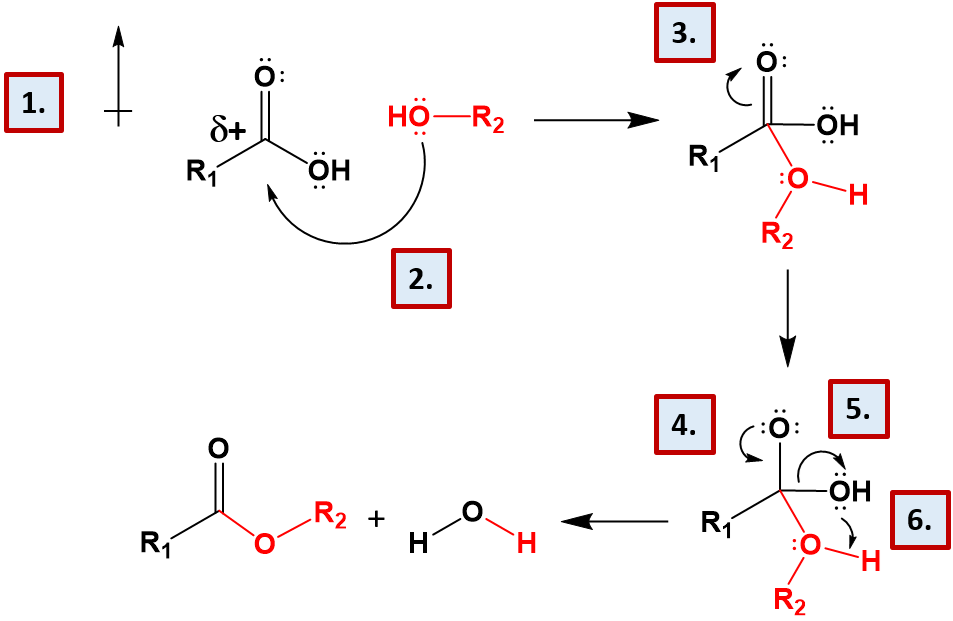
Ch103 Chapter 7 Chemical Reactions In Biological Systems Chemistry

How Chemical Reactions Form New Products Video Lesson Transcript Study Com

Pin By Classroom Chemist On School Sciences Tpt Teaching Resources Digestive System Activities Digestive System Worksheet Science Tpt

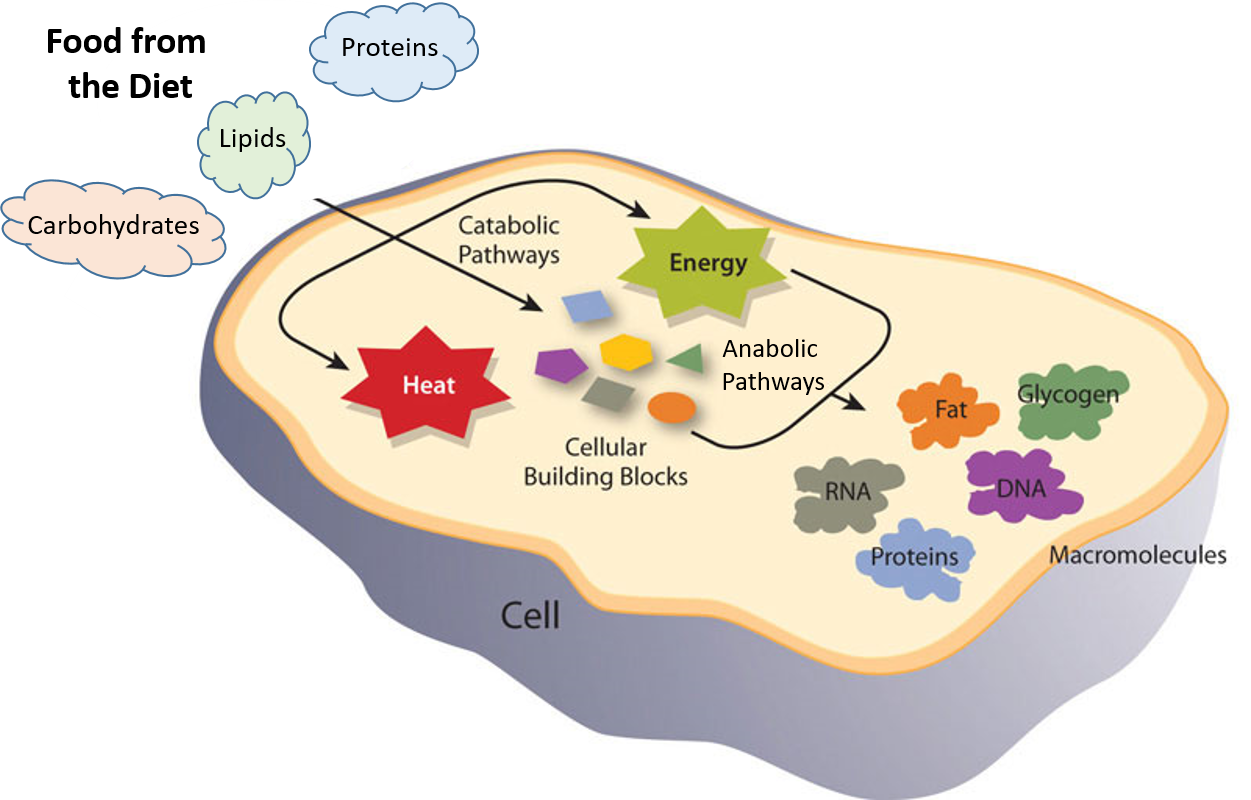
Pin On Macromolecules Paul Coates

Catalyst Speeds Up A Chemical Reaction By Lowering The Activation Energy Ea Of The Overall Reaction Chemistry Education Teaching Chemistry Energy Activities
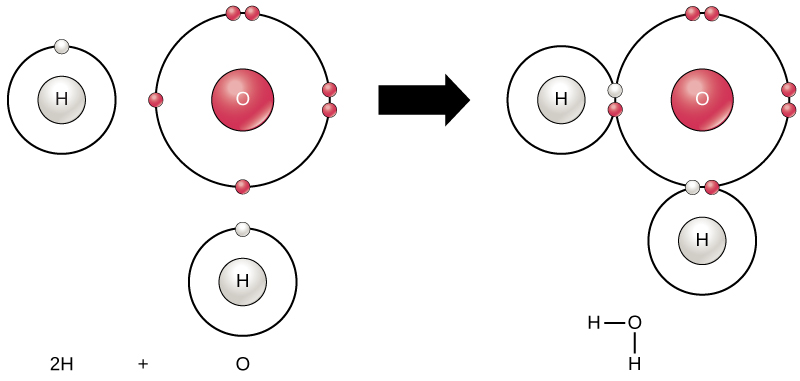
Chemical Reactions Biology For Majors I
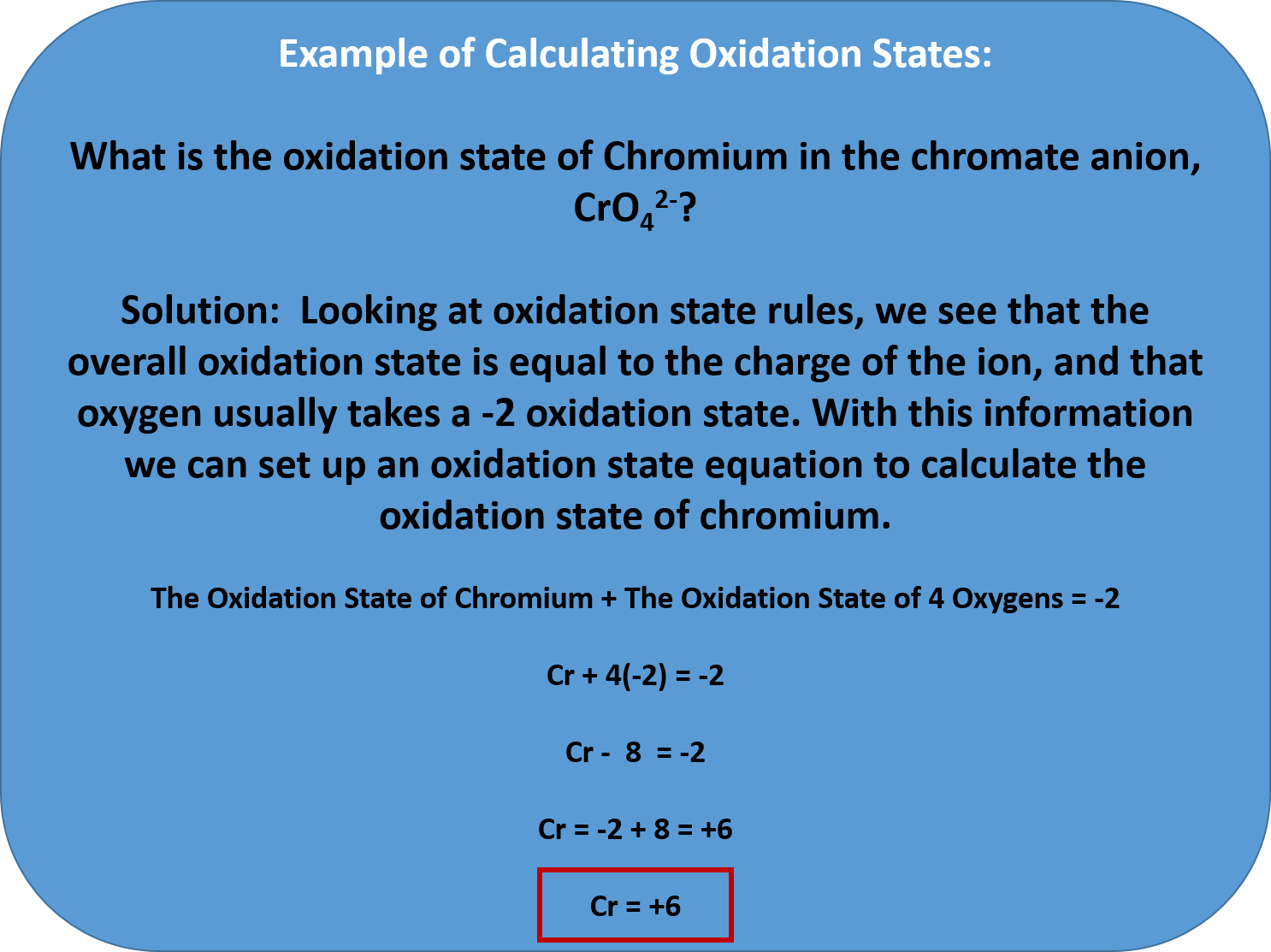
Ch103 Chapter 7 Chemical Reactions In Biological Systems Chemistry

Reactants Products What Are The Products In A Chemical Reaction Video Lesson Transcript Study Com

Chemistry Education Teaching Chemistry Physics And Mathematics

Chemical Reactions And Equations Ck 12 Foundation

Chemical Reactions Advanced Read Biology Ck 12 Foundation

Chemical Reactions Poster Teaching Chemistry Chemical Reactions Matter Science
Comments
Post a Comment